When sofosbuvir first reached the market it was not approved for patients with CKD (Chronic Kidney Disease) stages 4 and 5 which means patients with an eGFR of < 30 ml/min. This was based on the observations that:
- Sofosbuvir is rapidly metabolised to GS-331007 (t1/2 0.4 hours), and
- GS-331007 (t1/2 27 hours) has renal excretion, and
- In the context of a reduced eGFR the AUC (Area Under The Curve) for this metabolite rises substantially
The concern was that the higher levels of GS-331007 might be toxic. This presents a real problem in countries that are dependent on generic DAA medication for HCV treatment because all these regimens are based on Sofosbuvir. There are currently no generic versions of Mavyret or Zepatier.
The short story is that experimentation has shown the following:
- The higher levels of the GS-331007 in patients with CKD are not toxic
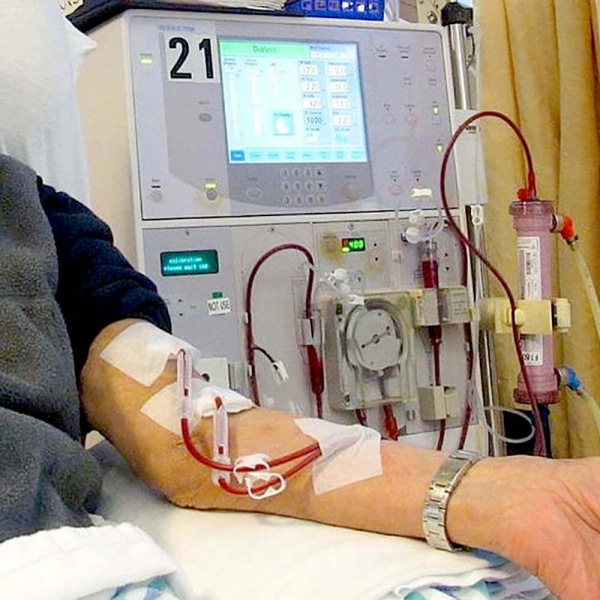
- GS-331007 is cleared by haemodialysis or by the remaining GFR
- Reduced dose sofosbuvir (ie 200 mg daily) results in poor SVR12 rates (<50%) in patients with CKD
- Full dose sofosbuvir (ie 400mg daily) results in normal SVR12 rates (~95%) in patients with CKD +/- dialysis without problematic side effects
Here is a non-exhaustive list of published studies showing the use of full-dose sofosbuvir in patients with CKD is safe and effective:
- AASLD 2018 - Sofosbuvir/Velpatasvir for 12 Weeks Is Safe and Effective in Patients Undergoing Dialysis
- Hepatology Communications 2017 - Sofosbuvir‐based regimens for the treatment of chronic hepatitis C in severe renal dysfunction
- J Clin Transl Hepatol. - Sofosbuvir Use in the Setting of End-stage Renal Disease: A Single Center Experience
- Kidney Int Rep. - Hemodialysis Patients Treated for Hepatitis C Using a Sofosbuvir-based Regimen
- Indian J Gastroenterol. - Efficacy and safety of sofosbuvir-based regimens in chronic hepatitis C patients on dialysis
- Liver Int. - Sofosbuvir-based treatment is safe and effective in patients with chronic hepatitis C infection and end-stage renal disease: a case series
- J Hepatol. - Pharmacokinetics, safety and efficacy of a full dose sofosbuvir-based regimen given daily in hemodialysis patients with chronic hepatitis C
So, although the current EASL and AASLD guidelines suggest using an alternative to Sofosbuvir, these guidelines were written by people in live in countries with the luxury of having alternatives.
Where alternatives to Sofosbuvir are not available there is sufficient evidence in the public domain indicating it is safe to use Sofosbuvir in CKD, which, to me, makes it ethical given the usual benefits exceed the risks equation we all use to guide our clinical practice.
For those who are interested why here is the explanation.
Sofosbuvir is not an active drug. It is a prodrug that requires activation. This activation happens as follows:
- The concentration gradient of the Sofosbuvir prodrug depends almost entirely on the Cmax (rather than the AUC) so, in line with usual pharmacokinetics, the higher the dose administered the higher the Cmax
- The higher the concentration, the more Sofosbuvir penetrates the target cells as there is a higher concentration gradient driving this influx
- Once the sofosbuvir is intracellular it is cleaved and phosphorylated into the active entity which is a polar charged molecule trapped in the cell
In other words, Sofosbuvir is like Gentamicin where it is the Cmax peak that determines efficacy, not the AUC.
Reducing the administered Sofosbuvir dose
- reduces the Cmax, which
- reduces the concentration gradient driving it into target cells, which
- reduces the intracellular levels of the active cleaved/phosphorylated form and in turn
- reduces the time that this level exceeds the EC50
This also explains the lower efficacy in cirrhotic patients. Fibrous scar tissue has a reduced blood supply, this reduces the effective concentration gradient driving the Sofosbuvir into cells due to a dynamic depletion effect - although the concentration in the blood is the same, the small quantity of blood soon runs out of Sofosbuvir...
So while for many renally cleared drugs (including Ribavirin) we do need to decrease the dose in CKD we do not want to do this with Sofosbuvir. Fortunately for patients, the circulating metabolite that is renally cleared (GS-331007) has been proven to be non-toxic so the fact it will be circulating at higher levels in patients with CKD can be safely ignored. This metabolite is cleared by either a low eGFR or dialysis.
Unfortunately, I can't share it here at the moment, because it is in publication as we speak, but I am aware of a large multicentre trial in patients with CKD and haemodialysis who were given full dose Sofosbuvir 400 mg / Daclatasvir 60 mg daily and achieved 98% SVR12.