Displaying items by tag: hepatitis c
Patient Rights vs Patent Rights
The thing with Hep C is that it's not about anonymous statistics, it's about real people. Here's the story of one patient, and his wife's journey through Interferon and failure and then to self initiated treatment and cure via the parallel import of generic DAA medications that was published in the Sydney Morning Herald and The Age:
Here is a letter to the editor that did not make the cut
Sunday 27th September 2015
Dear Editor
(Ref: Hepatitis C drug buyers club aims to set up new source of support)
In 1961 JFK uttered the immortal lines "ask not what your country can do for you - ask what you can do for your country".
With the passage of time, the idea we should all put something back in seems increasingly lost.
We have at our fingertips the tools to rid the world of Hepatitis C and are separated from that only by corporate avarice.
Gilead Sciences are asking for more than the entire annual PBS medications budget, used to treat all Australians for all diseases, to treat a single disease forecast to kill half as many people as breast cancer by 2030.
If this medication pricing trend continues unabated you can foresee the day we invent a cure for cancer, but people still die because only a fortunate few can afford access.
Parallel importing is a tool that has been used before to level the playing field, most notably around the pricing of HIV medications. $1000 a tablet for something that costs $1 to produce and is available overseas for $10 does not make sense.
It’s time to draw a line in the sand and make it clear patient rights deserve equal protection to patent rights.
Kind Regards
Dr James Freeman
The Minimum Cost To Cure Hepatitis C - Revisited
A patient posted a link that contained a powerpoint presentation from Dr Andrew Hill, PhD. I asked Dr Hill if I could post it here and he said yes.
I turned that into a very quick YouTube movie, so you will probably have to pause it to read it. The attachments include the original PPT (and a PDF version) and two recent papers. Use the readmore to get to them.
Hep C drugs queue just gets longer
Hepatitis Australia is using new data released from the Kirby Institute today showing only one per cent of people with hep C received treatment last year to push the government to list new hep C drugs without delay.
"It's time for action," said Kevin Marriott, Hepatitis Australia Acting CEO. "It's time of the federal government to make new therapies widely available, increase liver clinic capacity, upscale hepatitis C treatment and prevention programs and transform the lives of thousands of Australians."
New surveillance data from the Kirby Institute estimated some 230,470 people had chronic hepatitis C infection at the end of 2014. Around 80 percent had early to moderate fibrosis and 19 percent had severe fibrosis or hepatitis C related cirrhosis.
The estimated number of people with severe liver disease/hepatitis C related cirrhosis has more than doubled in ten years, according to the data.
Head of the viral hepatitis clinical research program at the Kirby Institute Professor Greg Dore said that without significant improvement in hepatitis C treatment rates, Australia would see a 245 percent increase in the rates of liver cancer and 230 percent increase in hep C-related deaths by 2030.
"Thousands of Australians are queuing up waiting for new medicines to be PBS listed. These treatments provide one of the great breakthroughs in clinical medicine in recent decades, with enormous potential to improve the lives of people living with hepatitis C," Professor Dore said.
Four new hep C medicines - Gilead's Sovaldi (sofosbuvir) and Harvoni (ledipasvir/sofosbuvir), BMS's Daklinza (daclatasvir)and AbbVie's Viekira Pak (paritaprevir with ritonavir, ombitasvir and dasabuvir plus ribavirin) - have been recommended for PBS listing but price negotiations with sponsors are ongoing. Professor Dore said previously, he suspected listing is more likely for sometime in 2016.
Mr Marriott said there is compelling evidence for the new medicines to be listed without delay before people progress to serious liver disease and die.
"Interferon-free therapies will allow the vast majority of people living with the hepatitis C virus to be cured, even where treatment has failed previously and without the terrible side-effects of existing treatments."
Michelle Lam
Originally published in Pharma in Focus. Reproduced with permission.
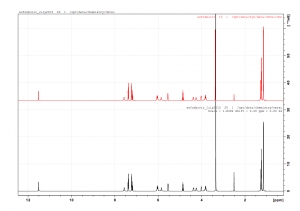
Sofosbuvir Reference NMR Comparison
Today we compared a new batch of Sofosbuvir and Ledipasvir to our reference sample data and found it consistent.
It's reassuring to see the consistency.
WHO moves to improve access to lifesaving medicines for hepatitis C
GENEVA - WHO today published the new edition of its Model List of Essential Medicines which includes ground-breaking new treatments for hepatitis C.
http://www.who.int/mediacentre/news/releases/2015/new-essential-medicines-list/en/