Displaying items by tag: generic hepatitis c
Confirmed: Hep C treatment saves lives
So back in late 2017 the Cochrane Collaboration made the bold claim that treatment with Direct Acting Antivirals did not have any measurable impact on clinical outcomes. While the medical profession as a whole expressed a large and collective WTF sentiment new research published in the Lancet - "Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study" - puts this question to bed. The full text of the article and the editorial comment are attached, but here is the summary:
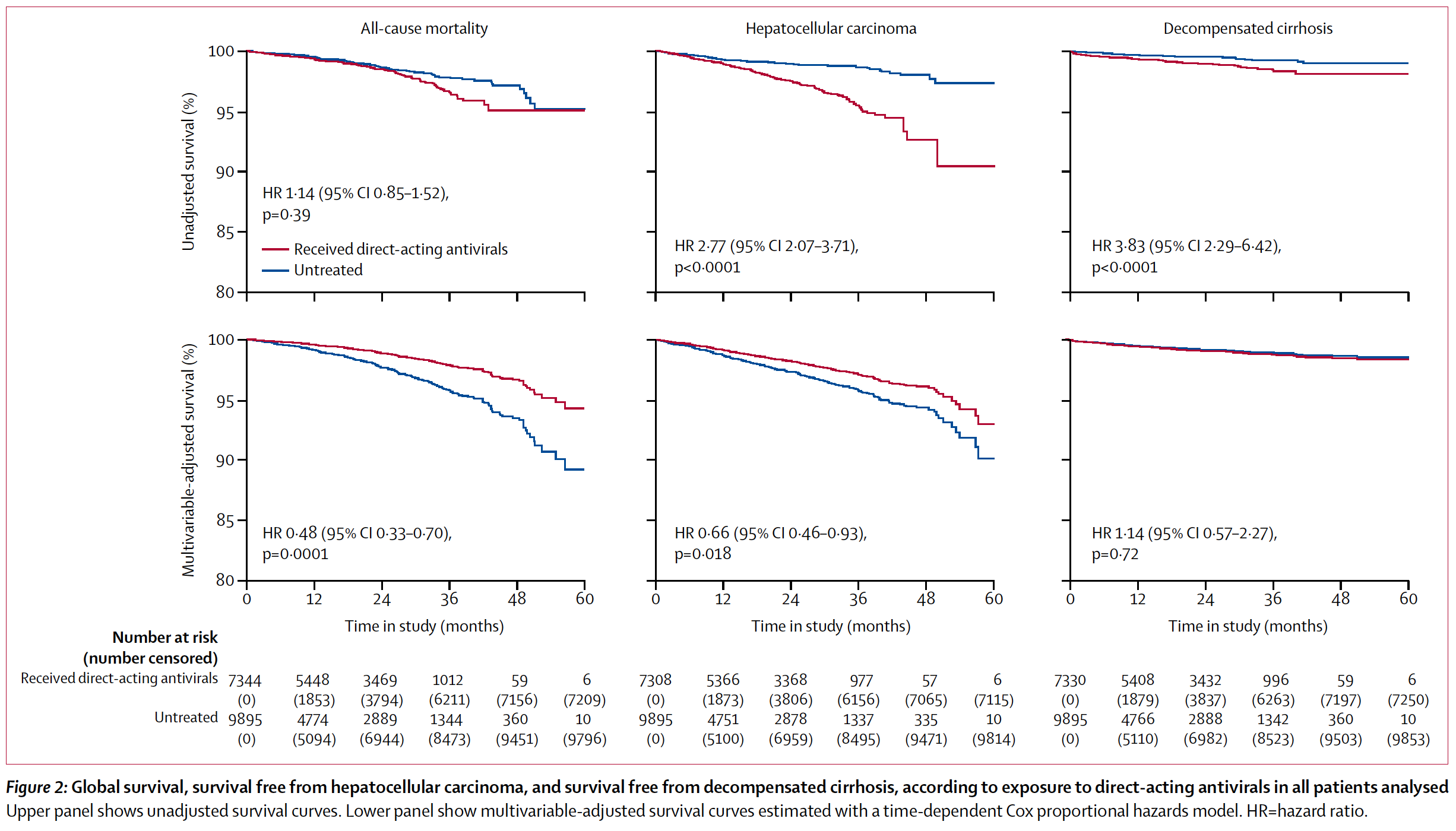
Findings of adjusted multivariable analyses showed that exposure to direct-acting antivirals significantly reduced all-cause mortality (hazard ratio [HR] 0·48, 95% CI 0·33–0·70; p=0·0001), liver-related mortality (0·39, 0·21–0·71; p=0·0020), non-liver-related mortality (0·60, 0·36–1·00; p=0·048), and hepatocellular carcinoma (0·66, 0·46–0·93; p=0·018), but not decompensated cirrhosis (1·14, 0·57–2·27; p=0·72). These associations persisted when the analysis was restricted to patients with cirrhosis who achieved sustained virological response. By contrast, failure to achieve sustained virological response was associated with increased risk for hepatocellular carcinoma (HR 2·23, 95% CI 1·37–3·64; p=0·0012).
Bioequivalent pharmacokinetics for generic and originator hepatitis C direct-acting antivirals
They say (who are they anyway?) "If it seems to good to be true it probably is". When it comes to generic Hepatitis C medication some people struggle with the idea that something like Harvoni® with a list price of $94,000 USD could be manufactured for under $1000 USD. Surely if the original costs $94,000 there must be something wrong with the more affordable generic. It's cheaper, they must have cut corners or something...
Well, the reality is that it does not cost very much to make a tablet of Harvoni® (about $1 to $2). Let's face it $1000 and is a lot of money for a single tablet, after all it will buy you a new iPhone and that's a lot more complicated than a pill. The $31,000 for a bottle of Harvoni® is the same as the price of a new car. And while $94,000 won't buy you a whole house in most places it is actually 1/2 the median price for a house in the USA. 3 Bottles of pills cost the same as 1/2 a house? Really?
Ok, I hear you say "So now I can understand that the price of Harvoni® represents outrageous price gouging, but that still does not prove the less expensive generics meet the required quality standard now does it?"
Fair point, and one that is answered by a set of tests called "bioequivalence" (BE). Bioequivalence testing starts with baseline things like high-quality factories, using high-quality active ingredients and putting them together into tablets under what is called GMP (Good Manufacturing Practice). GMP is a series of processes that provision QC (Quality Control) / QA (Quality Assurance). Ok, so now we have some tablets. They are in a nice bottle and have been made by experts. These experts have put their brand name on them but... that still does not prove those tablets are going to work the same. Why?
The why is because for a generic tablet to work the same as the originator it needs to have more than the same APIs (Active Pharmaceutical Ingredients) in the correct weights. These tablets also need to be formulated correctly to ensure that when a patient takes them (be it generic or originator) the product delivers the same blood levels to the patients. So how do we do that?
It's actually really simple. We recruit 24-60 volunteers. We give 1/2 of them the originator medication and the other 1/2 the generic. We then make 20 measurements of the blood levels over the next 12 hours. Then we wait a week for the drugs to wash out. Then we repeat the testing but the patients who had the generic first time around now get the originator, and the patients who got the originator now get the generic. This may then be repeated again (fully replicated).
A product is deemed to be bioequivalent if the blood levels at all time points demonstrate the statistical confidence interval (CI) fall within the range 80% - 125%.
Oh, I hear you say, "Does that mean a bioequivalent generic can have up to 20% less or 25% more drug in it?"
Not at all, because I said "confidence interval".
Ok, I hear you say, "WTF is a confidence interval?"
Glad you asked, although your eyes may glaze over!
Consider a coin toss. We toss it once and get a head. So our results are 100% heads. The actual result should be 50% heads (unless the coin is loaded). The confidence interval (technically a binomial confidence interval) is a measure of how confident we are in that 100% result. The confidence interval, in this case, is 2.5% - 100% so you can see we are not very confident. You may note that this range is wide but it does contain the actual value. Now if we toss the coin 3 times we might get 1 head and 2 tails. So we now have 33% heads and the confidence interval is 8.4% to 90%. The result is more realistic, the confidence interval is a little narrower but... Say we toss it 10 times and get 4 heads and 6 tails. Now we have 40% heads and the confidence interval is 12% to 73%. And finally, we toss the coin 100 times and get 49 heads and 51 tails. Now we have 49% heads and the confidence interval is 39%-59%. You can play with other numbers here: http://statpages.info/confint.html
What I want you to notice are 2 things. The more tests we do the more accurate the actual results become and the narrower the confidence interval becomes. With the 100 toss trial the 39% lower confidence interval is 22% lower than the expected 50% and the 59% upper confidence interval is 18% higher so it spans the confidence interval range on the actual value of 50% from 78% - 118% - you may note that this is similar to the bioequivalence threshold of 80% (-20%) to 125% (+25%).
What this means in practical terms is that when we test for bioequivalence the actual values at any point need to be very close together to meet the statistical confidence interval criteria and we need to do a lot of tests to narrow the CI range down. Here's what it actually looks like and as you can see these generic products are very very similar to the originator product. The key values of Cmax (Concentration maximum) and AUC (Area Under the Curve and a measure of total absorption) vary only by a low single digit % amount. Some are a little higher, some are a little lower. All are very similar in absolute and statistical terms.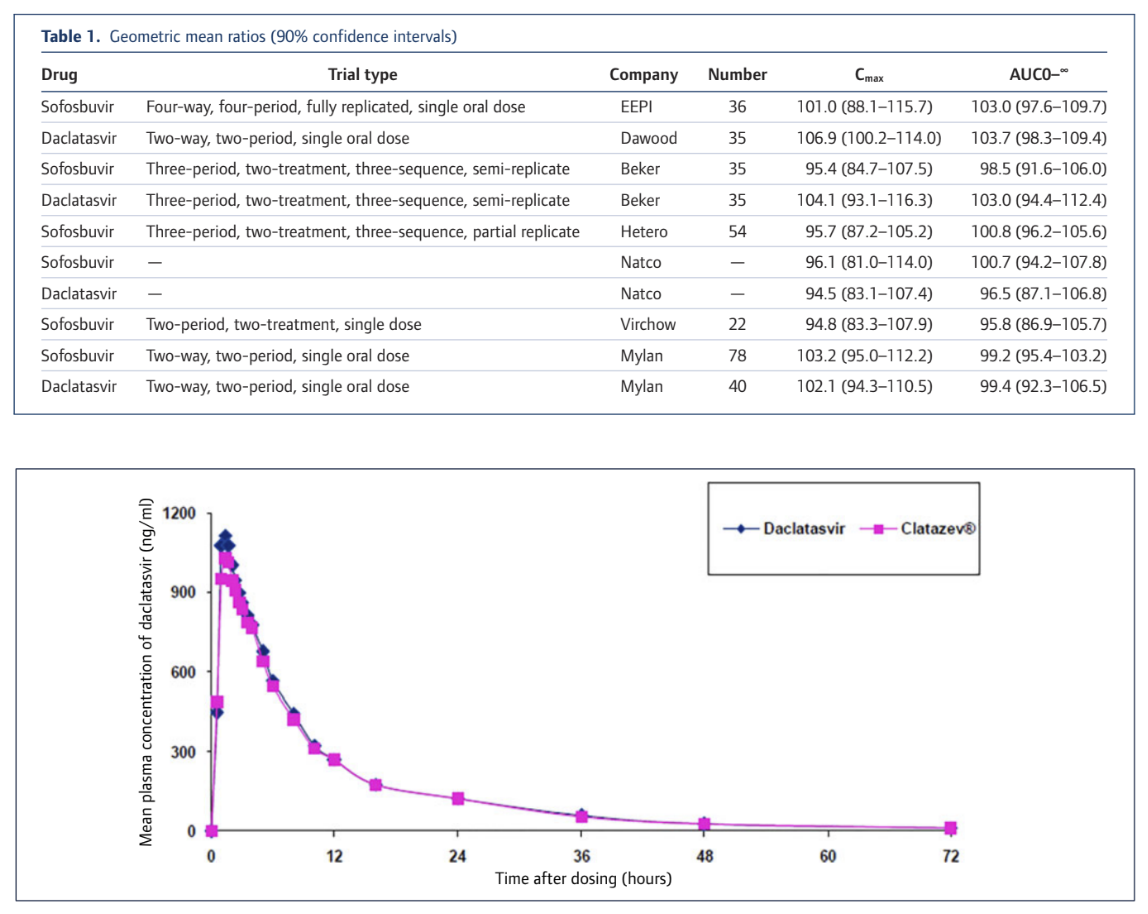
The full details of our collation of BE testing results are available in the attached article from the Journal of Virus Eradication or online here: http://viruseradication.com/journal-details/Bioequivalent_pharmacokinetics_for_generic_and_originator_hepatitis_C_direct-acting_antivirals/
We have the power to FixHepC, but the real question is...
We have the power to FixHepC, but the real question is... Do we have the willpower?
This was how Dr James Freeman ended his presentation at the World Hepatitis Summit in Brazil.
You can watch the full presentation on YouTube.
In essence, if we applied the same effort to HCV as we do to HIV this epidemic could be over within a single year. Currently, we treat 18 million patients with one year's supply of multiple antiviral drugs. That would translate to 72 million 3 month treatment courses - enough to treat every man woman and child with HCV.
We still have the enormous challenge of finding those patients in time, but we clearly have the funding capacity and the industrial capacity to get it done.
One year, that's all it would take.
The slides are attached below:
Slides from Dr James Freeman's Generics Presentation at the World Hepatitis Summit 2017



